
Per VLP Therapeutics:
Gaithersburg, MD — US-based biotech company VLP Therapeutics, Inc. (VLPT) announced on December 27 that it has signed an agreement for an investment of US$21 million in a Series A-1 round from six investors, consisting of two new investors: Nobelpharma Co., Ltd.and MUFG Bank, Ltd., and four existing investors: Sojitz Corporation, MIYAKO Capital Co., Ltd., Mr. Robert G. Hisaoka and SK Impact Fund, LLC.
With this funding, VLPT aims to further accelerate the research and development of a cancer treatment vaccine as well as prophylactic vaccines against malaria, dengue, etc. and move into clinical trials at the earliest date possible. This is an additional investment following US$16 million raised in a Series A round in March 2021.
“I have long committed to the research and development of vaccines against cancer and infectious diseases so the people across the globe can lead normal lives,” says Wataru Akahata, CEO and co-founder of VLPT. “We were fortunate enough to be able to raise funding in March to facilitate our cancer treatment vaccine R&D. This additional funding will now allow us to further accelerate our other R&D efforts in infectious diseases area as well. This means a lot as it helps us to push our scientific endeavors forward at much faster pace, enabling us to get one step closer in making a greater social impact.”
About VLP Therapeutics: VLP Therapeutics, Inc. (VLPT), co-founded in 2013 by Drs. Wataru Akahata, Ryuji Ueno, and Sachiko Kuno, is a Gaithersburg, MD-based biotech company with a mission to address unmet medical needs worldwide and expand the frontiers of vaccine treatment. Led by CEO Akahata VLPT is currently engaged in research and development of a cancer treatment vaccine as well as prophylactic vaccines against malaria, dengue, etc. using VLPT’s proprietary platform technologies.
– Two-dose primary regimen of NVX-CoV2373 demonstrated cross-reactive immune responses against Omicron (B.1.1.529) and other variants
– Third dose produced increased immune responses comparable to or exceeding levels associated with protection in Phase 3 clinical trials, with a 9.3-fold IgG rise and a 19.9-fold ACE2 inhibition increase after booster dose
– Immune responses in adolescents were 2- to 4-fold higher than adults against broad array of variants of interest and variants of concern
– Development of Omicron-specific vaccine on track for initiation of GMP manufacturing in early January
– Company to host investor conference call today from 4:30 – 5:00 pm ET
GAITHERSBURG, Md., Dec. 22, 2021 /PRNewswire/ — Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializing next-generation vaccines for serious infectious diseases, today announced initial data evaluating the immune response of its COVID-19 vaccine, NVX-CoV2373, against the Omicron variant as well as additional data from its ongoing Phase 2 boost study. New results demonstrate broad cross-reactivity against Omicron and other circulating variants from a primary 2-dose regimen, with responses that increased following a third dose at six months.
Immune responses included the following:
- Anti-spike IgG titers after Dose 3 increased 5.4-fold (prototype) to 9.3-fold (Omicron) from peak responses seen after 2-dose primary vaccination.
- This represents a 61.1-fold (prototype) and a 73.5-fold (Omicron) increase from prior to the Dose 3 boost.
- ACE2-inhibition titers increased 6-fold (prototype) to 19.9-fold (Omicron) compared to peak responses following 2-dose primary series, representing a 54.4-fold (prototype), a 24.4-fold (Delta) and a 36.3-fold (Omicron) increase from prior to the booster.
- Wild-type neutralization responses were observed after 2 doses for prototype, Delta and Omicron. Significant increases were observed after boosting, with titers for Delta and Omicron comparable to levels associated with protection in U.S. and Mexico and U.K. Phase 3 studies.
- After 2 doses, Omicron wild-type neutralization was <4-fold lower than prototype, suggesting that both a booster dose as well as an Omicron-specific vaccine may be beneficial.
Further, data from the pediatric expansion of Novavax’ PREVENT-19 Phase 3 trial in the U.S. and Mexico showed robust immune responses in adolescents, including increased IgG and receptor inhibition titers against a wide array of variants, including Omicron, following a 2-dose series. Responses in adolescents were 2- to 4-fold higher than adults against all evaluated variants.
“In the midst of an evolving pandemic, NVX-CoV2373 showed strong immune responses against Omicron and other circulating variants. We are encouraged that boosted responses against all variants were comparable to those associated with high vaccine efficacy in our Phase 3 clinical trials, suggesting that NVX-CoV2373 can play an important role in the ongoing fight against new variants,” said Gregory M. Glenn, President of Research and Development, Novavax. “Given the continued evolution of the coronavirus, the development of an Omicron vaccine could be necessary. Novavax has cloned, expressed and characterized the Omicron spike protein vaccine and will soon enter the GMP-phase of production. We expect to begin clinical studies in the first quarter of 2022.”
As part of an ongoing study, a single booster dose of 5 µg SARS-CoV-2 rS with 50 µg Matrix-M™ adjuvant was administered to healthy adult participants approximately six months after their primary 2-dose vaccination series. Multiple assays were used to evaluate immune responses against SARS-CoV-2 twenty-eight days following the booster dose.
Safety reporting of reactogenicity events showed an increasing trend across all 3 doses of NVX-CoV2373, reflecting the increased immunogenicity seen with a third dose. Following the booster, local and systemic reactions were generally short-lived with a median duration of approximately 2 days. The incidence of Grade 3 or higher events remained relatively low. Medically attended adverse events (MAAEs), potentially immune-mediated medical conditions (PIMMCs), and severe adverse events (SAEs) occurred infrequently following the booster dose and were balanced between vaccine and placebo groups.
The major findings, detailed in ‘Immunogenicity and Safety Following a Homologous Booster Dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): A Phase 2 Randomized Placebo-Controlled Trial,’ will be submitted for peer-review publication and are expected to be available online at https://www.medrxiv.org/ in the coming days.
Conference Call
Novavax will host a conference call for investors today at 4:30 p.m. ET. The dial-in numbers for the conference call are (877) 870-4263 (Domestic) or (412) 317-0790 (International). Participants will be prompted to request to join the Novavax, Inc. call. A replay of the conference call will be available starting at 7:30 p.m. ET on December 22, 2021 until 11:59 p.m. ET on December 31, 2021. To access the replay by telephone, dial (877) 344-7529 (Domestic) or (412) 317-0088 (International) and use passcode 6207101.A webcast of the conference call can also be accessed on the Novavax website at novavax.com/events. A replay of the webcast will be available on the Novavax website until March 22, 2022.
About NVX-CoV2373
NVX-CoV2373 is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2, the virus that causes COVID-19 disease. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike (S) protein and is formulated with Novavax’ patented saponin-based Matrix-M™ adjuvant to enhance the immune response and stimulate high levels of neutralizing antibodies. NVX-CoV2373 contains purified protein antigen and can neither replicate, nor can it cause COVID-19.Novavax’ COVID-19 vaccine is packaged as a ready-to-use liquid formulation in a vial containing ten doses. The vaccination regimen calls for two 0.5 ml doses (5 mcg antigen and 50 mcg Matrix-M adjuvant) given intramuscularly 21 days apart. The vaccine is stored at 2°- 8° Celsius, enabling the use of existing vaccine supply and cold chain channels. The current assigned shelf life of the vaccine is 9 months.
Novavax has established partnerships for the manufacture, commercialization and distribution of NVX-CoV2373 worldwide.
About the NVX-CoV2373 Phase 3 trials
NVX-CoV2373 is being evaluated in two pivotal Phase 3 trials.A trial conducted in the U.K. with 14,039 participants was designed as a randomized, placebo-controlled, observer-blinded study and achieved overall efficacy of 89.7%. The primary endpoint was based on the first occurrence of PCR-confirmed symptomatic (mild, moderate or severe) COVID-19 with onset at least 7 days after the second study vaccination in serologically negative (to SARS-CoV-2) adult participants at baseline. Full results of the trial were published in the New England Journal of Medicine (NEJM).
PREVENT-19, a trial in the U.S. and Mexico, with 25,452 participants, achieved 90.4% efficacy overall. It was designed as a 2:1 randomized, placebo-controlled, observer-blinded study to evaluate the efficacy, safety and immunogenicity of NVX-CoV2373. The primary endpoint for PREVENT-19 was the first occurrence of PCR-confirmed symptomatic (mild, moderate or severe) COVID-19 with onset at least 7 days after the second dose in serologically negative (to SARS-CoV-2) adult participants at baseline. The statistical success criterion included a lower bound of 95% CI >30%. The key secondary endpoint is the prevention of PCR-confirmed, symptomatic moderate or severe COVID-19. Both endpoints were assessed at least seven days after the second study vaccination in volunteers who had not been previously infected with SARS-CoV-2. It was generally well-tolerated and elicited a robust antibody response in both studies. Full results of the trial were published in NEJM.
About Matrix-M™ Adjuvant
Novavax’ patented saponin-based Matrix-M™ adjuvant has demonstrated a potent and well-tolerated effect by stimulating the entry of antigen-presenting cells into the injection site and enhancing antigen presentation in local lymph nodes, boosting immune response.About Novavax
Novavax, Inc. (Nasdaq: NVAX) is a biotechnology company that promotes improved health globally through the discovery, development and commercialization of innovative vaccines to prevent serious infectious diseases. The company’s proprietary recombinant technology platform harnesses the power and speed of genetic engineering to efficiently produce highly immunogenic nanoparticles designed to address urgent global health needs. NVX-CoV2373, the company’s COVID-19 vaccine, received Conditional Marketing Authorization from the European Commission, Emergency Use Listing from the World Health Organization, Emergency Use Authorization in Indonesia and the Philippines, and has been submitted for regulatory authorization in multiple markets globally. NanoFlu™, the company’s quadrivalent influenza nanoparticle vaccine, met all primary objectives in its pivotal Phase 3 clinical trial in older adults. Novavax is currently evaluating a COVID-NanoFlu combination vaccine in a Phase 1/2 clinical trial, which combines the company’s NVX-CoV2373 and NanoFlu vaccine candidates. These vaccine candidates incorporate Novavax’ proprietary saponin-based Matrix-M™ adjuvant to enhance the immune response and stimulate high levels of neutralizing antibodies.For more information, visit www.novavax.com and connect with us on Twitter, LinkedIn, Instagram and Facebook.
Forward-Looking Statements
Statements herein relating to the future of Novavax, its operating plans and prospects, its partnerships, the ongoing development of NVX-CoV2373, the scope, timing and outcome of future regulatory filings and actions, including Novavax’ plans to submit a complete CMC data package to the U.S. FDA by the end of the year, and the efficacy, safety and intended utilization of NVX-CoV2373 are forward-looking statements. Novavax cautions that these forward-looking statements are subject to numerous risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. These risks and uncertainties include challenges satisfying, alone or together with partners, various safety, efficacy, and product characterization requirements, including those related to process qualification and assay validation, necessary to satisfy applicable regulatory authorities; difficulty obtaining scarce raw materials and supplies; resource constraints, including human capital and manufacturing capacity, on the ability of Novavax to pursue planned regulatory pathways; challenges meeting contractual requirements under agreements with multiple commercial, governmental, and other entities; and those other risk factors identified in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Novavax’ Annual Report on Form 10-K for the year ended December 31, 2020 and subsequent Quarterly Reports on Form 10-Q, as filed with the Securities and Exchange Commission (SEC). We caution investors not to place considerable reliance on forward-looking statements contained in this press release. You are encouraged to read our filings with the SEC, available at www.sec.gov and www.novavax.com, for a discussion of these and other risks and uncertainties. The forward-looking statements in this press release speak only as of the date of this document, and we undertake no obligation to update or revise any of the statements. Our business is subject to substantial risks and uncertainties, including those referenced above. Investors, potential investors, and others should give careful consideration to these risks and uncertainties.
Per the State of Maryland:
ANNAPOLIS, MD—Governor Larry Hogan today announced that, according to official CDC data, 90% of all Marylanders 18 and older have now received at least one dose of a COVID-19 vaccine. This is a major milestone for the state’s vaccination campaign.
“Even as we continue to urge booster shots, we are also very focused on getting the remaining unvaccinated individuals vaccinated,” said Governor Hogan. “As part of our commitment to leave no arm behind, we will work to get that last remaining 10% vaccinated. This continues to be the single most important thing you can do to protect yourself, your family, and your fellow Marylanders.”

Booster Eligibility Open To All Marylanders 16 and Older. On Thursday, the governor announced that the state has expanded eligibility for booster shots to include all Marylanders 16 and older. To date, the state is reporting 1,236,872 booster shots administered. Nearly 60% of the state’s eligible seniors have received a booster shot.

Find a Vaccine Clinic. To find a clinic, visit covidvax.maryland.gov or call the state’s multilingual call center, available seven days a week, at 1-855-MD-GOVAX (1-855-634-6829).
Request a Vaccine Clinic. Businesses, schools, organizations, or community groups can request a GoVAX mobile clinic for their organization at governor.maryland.gov/govaxmobile or call 1-855-MD-GOVAX (1-855-634-6829).
MCPS has been doing targeted outreach to school families to help them make appointments. More appointments for the weekend clinics will be coming online later this morning. We expect supply to ramp up quickly but currently, supply is limited and demand is high.
— Montgomery County DHHS (@MoCoDHHS) November 5, 2021
11/5 6:30am Update Seen Above: “More appointments for the weekend clinics will be coming online later this morning. We expect supply to ramp up quickly but currently, supply is limited and demand is high.”
MCPS is partnering with the county’s Department of Health and Human Services and Holy Cross Hospital to offer a number of free vaccination clinics for children at schools and county sites once the vaccine is available. Parents are urged to be patient as initially there will not be enough supply to vaccinate all eligible children immediately.
Other family members who have not received a COVID-19 vaccination or who need a Pfizer booster, will be able to do so at these clinics as well.
2/3 of the doses for children 5 to 11 will be provided by pharmacies, pediatricians, other healthcare providers and about 1/3 will be provided by the health department.
Residents are encouraged to take advantage of the numerous sources that are providing vaccinations.
Below you’ll see vaccination sites, as provided by the MCPS website.
MCPS COVID-19 VACCINATION WEEKEND SITES
Friday Clinics at County sites, November 5 – 4 p.m. to 8 p.m.
Vaccine: Pfizer 5-11
| Location | Schedule an Appointment |
|---|---|
| Dennis Ave Health Center | Schedule |
| East County Recreation Center | Schedule |
| Montgomery College – Germantown | Schedule |
Saturday Clinics, November 6 – 10 a.m. to 6 p.m.
Vaccine: Pfizer 5-11
| School | Address | Schedule an Appointment |
|---|---|---|
| Banneker Middle School | 14800 Perrywood Drive, Burtonsville, MD 20866 | Schedule |
| Francis Scott Key Middle School | 910 Schindler Drive, Silver Spring, MD 20903 | Schedule |
| Argyle Middle School | 2400 Bel Pre Road, Silver Spring, MD 20906 | Schedule |
| Neelsville Middle School | 11700 Neelsville Church Road, Germantown, MD 20876 | Schedule |
| Eastern Middle School | 300 University Blvd E, Silver Spring, MD 20901 | Schedule |
| Gaithersburg Middle School | 101 Education Blvd, Gaithersburg, MD 20877 | Schedule |
Sunday Clinics, November 7 – 10 a.m. to 6 p.m.
Vaccine: Pfizer 5-11
| School | Address | Schedule an Appointment |
|---|---|---|
| Loiederman Middle School | 12701 Goodhill Road, Silver Spring, MD 20906 | Schedule |
| Montgomery Village Middle School | 19300 Watkins Mill Road, Montgomery Village, MD 20886 | Schedule |
| Julius West Middle School | 651 Great Falls Road, Rockville, MD 20850 | Schedule |
| Newport Mill Middle School | 11311 Newport Mill Road, Silver Spring, MD 20902 | Schedule |
| Silver Spring International Middle School | 313 Wayne Ave, Silver Spring, MD 20910 | Schedule |
| Redland Middle School | 6505 Muncaster Mill Road, Derwood, MD 20855 | Schedule |
Per Montgomery County:
We will offer COVID-19 vaccine doses to children ages 5 to 11 beginning Thursday, November 4. Appointments are required.
The vaccine is being distributed to local health departments, private medical practices, and pharmacies in Maryland.
Two thirds of the doses will be provided by pharmacies and health care providers and about one third will be provided by the Montgomery County Department of Health and Human Services (DHHS) .
Residents are encouraged to take advantage of the numerous sources that are providing vaccinations.
We received our first doses this week and we will continue to get more doses weekly. Initially, the number of doses will be limited so we urge parents to be patient. We will not be able to vaccinate every eligible child immediately. Check with your health care provider to see if they are offering vaccinations.
We estimate that we have more than 100,000 children in the age 5 to 11 group. It will take some time to fully vaccinate every eligible child who wants it.
Children ages 5 to 11 will get 2 doses of the Pfizer vaccine, 3 weeks apart. The pediatric vaccine is specially packaged and is NOT interchangeable with the Pfizer used for first, second and booster doses for those older than 12 years of age.
Many locations will offer children’s appointments
Check with your child’s health care provider
Check with your child’s health care provider. Many pediatricians, family medicine physicians, pharmacies and large retailers will offer vaccine appointments.
Check community run clinics
Starting Friday, November 5, 2021, our DHHS clinics will offer appointments for 5 to 11 year olds from 4:00 to 8:00 p.m.
You must make an appointment for your child to get their COVID-19 vaccine at a community clinic. We cannot accept walk-ups—no exceptions. We plan our clinics’ vaccine supply so no leftover doses are wasted at the end of the day.
If you need help making your child’s appointment, call the COVID-19 Call Center at 240-777-2982 (Monday to Friday, 9 a.m. to 6 p.m.).
Make a community clinic appointmentThese community clinics will offer appointments for children ages 5 to 11:
Our children’s clinics will have vaccine doses for children ages 5-11. They will not have the doses needed for other age groups. We will cancel children’s clinic appointments for anyone age 12 and older, including parents and older siblings.
Prepare for your child’s appointment
Have your child wear clothing with easy access to their upper arm. We will ask them to roll up their sleeve for their vaccine dose.
Arrive no more than 15 minutes early for your child’s appointment. Every child with an appointment is guaranteed to get their vaccination during the clinic they registered for.
More doses are coming
If you cannot get an appointment right away, please be patient. More vaccine will be coming to our health department (DHHS), as well as more providers, retailers, and pharmacies in the coming weeks.
When more vaccine doses are available, we will work with Montgomery County Public Schools (MCPS) to host clinics at some schools. These school clinics will offer evening and weekend hours.
Local health care providers with doses for ages 5 to 11
- Access Now Urgent Care
- AccessAbility MedCare, LLC
- Advanced Neighborhood Pediatrics, LLC – Clarksburg
- Advanced Neighborhood Pediatrics, LLC – Silver Spring
- Bethesda Pediatrics
- Brookville Pharmacy and Wellness Center
- Capital City Primary and Immediate Care
- Capitol Medical Group
- Casa Ruben, Inc.
- Coleman Pediatrics
- Complete Care For Kids
- Discovery Pediatrics, LLC – 600 Doses
- Friendship Pediatrics, PA
- Green Pharmacy LLC
- Hashim S. Hashim, MD
- Hirsch Pediatrics
- Holy Cross Hospital – Germantown
- Horizon Pediatric, Inc.
- International Pediatrics, PA – Gaithersburg
- John Choi, MD PC
- Kaiser Permanente – Gaithersburg Medical Center
- Kelly Goodman, NP and Associates, PC
- Kenwood Pediatrics Center
- Maryland Pediatric Care LLC
- Medstar Medical Group at Forest Hill
- MedStar Medical Group Pediatrics at Olney
- Midtown Pharmacy
- Montgomery Medical Clinic – KESSOUS
- Olney Pediatrics
- Park Pediatrics – Takoma Park
- Pediatric and Adolescent Care of Silver Spring
- Pediatric and Adolescent Care, PA
- Pediatric Associates of Montgomery County, PA – Olney
- Pediatric Associates of Montgomery County, PA – Rockville
- Pediatric Associates of Montgomery County, PA – Silver Spring
- Pediatric Associates of Montgomery County, PA – Wheaton
- Pediatric Care
- Pediatric Care Center
- Potomac Valley Pediatrics
- Prime Pediatrics
- Privia MG – Abdow Friendship Pediatrics
- Privia MG – Marshak Medical Group
- Procare Pharmacy and Cosmetic Beauty System, LLC
- Rainbow Pediatric Clinic
- Rehoboth Medical Center LLC
- Right Care Pediatrics
- Saba Pediatric Medicine, LLC
- Shady Grove Pediatric Associates
- Silver Spring Pediatrics
- Smita Parikh Mengers, MD FAAP
- Spring Pediatrics, Inc
- Sunshine Pediatrics
- Theodore F Wells-Green, MD
- Toseg Medical Center
- Virgo-Carter Pediatrics
- Washington International Pediatrics
- White Oak Pediatrics – Gaithersburg
- White Oak Pediatrics – Silver Spring
- Wonder Years Pediatrics
- Xpress Pediatrics Urgent Care Center

Novavax, a Gaithersburg-based biotechnology company dedicated to developing and commercializing next-generation vaccines for serious infectious diseases, and Serum Institute of India Pvt. Ltd. (SII), the world’s largest vaccine manufacturer by volume, today announced that the National Agency of Drug and Food Control of the Republic of Indonesia, or Badan Peng was Obat dan Makanan (Badan POM), has granted emergency use authorization (EUA) for Novavax’ recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M™ adjuvant. It will be manufactured by SII in India and marketed by SII in Indonesia under the brand name COVOVAX™.
“The first authorization of Novavax’ COVID-19 vaccine exemplifies our commitment to equitable global access and will fill a vital need for Indonesia, which despite being the fourth most populous nation on earth, continues to work to procure sufficient vaccine for its population,” said Stanley C. Erck, President and Chief Executive Officer, Novavax. “This also marks the first regulatory authorization worldwide of a protein-based COVID-19 vaccine based on Phase 3 clinical data demonstrating efficacy and a favorable safety profile. This is a landmark moment for Novavax and our partner, Serum Institute of India, and it is the first of many authorizations that Novavax expects in the coming weeks and months for our vaccine globally.”
Because the vaccine is stored at 2° to 8° Celsius, the use of existing vaccine supply channels with more traditional cold chain capabilities is possible, potentially increasing access in hard-to-reach areas and vaccination rates across the nation. Initial shipments into Indonesia are expected to begin imminently.
“Access to supply of a safe and highly effective vaccine, coupled with the ease of its distribution, should be a critical enabler to help Indonesiacontrol the current coronavirus outbreak,” said Adar Poonawalla, Chief Executive Officer, Serum Institute of India. “We continue to work with urgency to ensure the first protein-based COVID-19 vaccine option in Indonesia is available for all awaiting its arrival.”
Novavax and SII have already filed for authorization of Novavax’ COVID-19 vaccine in India and the Philippines, as well as for Emergency Use Listing (EUL) with the World Health Organization (WHO). Novavax recently also completed rolling submissions for authorization of the Novavax vaccine with regulatory agencies in the United Kingdom, European Union, Canada and Australia. Novavax expects to submit additional regulatory filings for its vaccine around the world as well as an additional supplemental filing for its vaccine for EUL with the WHO, shortly. Novavax expects to submit its complete package to the U.S. FDA by the end of the year.
Indonesia is a member of the Pharmaceutical Inspection Co-operation Scheme (PIC/S), a non-binding co-operative arrangement between more than 50 regulatory authorities, including those in the U.S., United Kingdom, European Union, Australia and Canada, in the field of Good Manufacturing Practice (GMP) of medicinal products for human or veterinary use. PIC/S aims at harmonizing inspection procedures worldwide through the development of common standards in the field of GMP and at facilitating cooperation and networking between competent authorities, regional and international organizations, thus increasing mutual confidence.
For additional information on COVOVAX, including the Summary of Product Characteristics, Prescribing Information and Important Safety Information, please visit: Indonesia National Agency of Drug and Food Control (Badan POM). This information will be posted in the coming days.
Authorized Use of Novavax’ Covid-19 Vaccine in Indonesia
Badan POM has issued Emergency Use Authorization (EUA) for Covovax /Recombinant Spike Protein of SARS-CoV-2 Virus 5 mcg to induce immunity against SARS-CoV-2 to prevent COVID-19 for adults 18 years old and above.
Important Safety Information
COVOVAX is contraindicated in persons who have hypersensitivity to the active substance or to any of the excipients of this vaccine.
About the NVX-CoV2373 Phase 3 Trials
NVX-CoV2373 is being evaluated in two pivotal Phase 3 trials: the PREVENT-19 trial in the U.S. and Mexico that demonstrated 100% protection against moderate and severe disease and 90.4% efficacy overall. It was generally well-tolerated and elicited a robust antibody response. It is also being evaluated in a trial in the U.K. that demonstrated efficacy of 96.4% against the original virus strain, 86.3% against the Alpha (B.1.1.7) variant and 89.7% efficacy overall.
NVX-CoV2373, Novavax’ Covid-19 vaccine, is a protein-based vaccine candidate engineered from the genetic sequence of the first strain of SARS-CoV-2, the virus that causes COVID-19 disease. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike (S) protein and is formulated with Novavax’ patented saponin-based Matrix-M™ adjuvant to enhance the immune response and stimulate high levels of neutralizing antibodies. NVX-CoV2373 contains purified protein antigen and can neither replicate, nor can it cause COVID-19.
Novavax’ COVID-19 vaccine is packaged as a ready-to-use liquid formulation in a vial containing ten doses. The vaccination regimen calls for two 0.5 ml doses (5 microgram antigen and 50 microgram Matrix-M adjuvant) given intramuscularly 21 days apart. The vaccine is stored at 2°- 8° Celsius, enabling the use of existing vaccine supply and cold chain channels.
About Matrix-M™ Adjuvant
Novavax’ patented saponin-based Matrix-M™ adjuvant has demonstrated a potent and well-tolerated effect by stimulating the entry of antigen-presenting cells into the injection site and enhancing antigen presentation in local lymph nodes, boosting immune response.
Content verified October 29, 2021, courtesy of Montgomery County.
Eligible people
If you are eligible, you may choose any of the available COVID-19 vaccines as your booster. If you want a specific vaccine as your booster, check that your vaccination site has it available.
| If you got this vaccine | Moderna or Pfizer series (2 shots) | Johnson & Johnson (1 shot) |
|---|---|---|
| When | Second shot at least 6 months ago | At least 2 months ago |
| In one of these groups |
|
Age 18 and older |
| You may receive any one of these vaccines as your booster shot |
|
|
Available locations
Items to bring to your appointment
Bring these items to your additional dose appointment
- your vaccination card – see how to get a copy of your record
- personal identification – see examples of identification
You will be required to self-certify underlying medical conditions and age.
You do not need a doctor’s note or medical history.
Are there risks of receiving an additional vaccine dose?
Limited information exists about the risks of receiving an additional dose of vaccine. Ongoing research is looking at the safety and benefit of additional doses of COVID-19 vaccines.
So far, reactions reported after a third dose of an mRNA vaccine were similar to those for first and second doses. The most common side effects reported have been fatigue and pain at the injection site. Overall, most side effects have been mild to moderate.

- Filing marks first protein-based COVID-19 vaccine submitted to MHRA for authorization
- All modules required for regulatory review, including CMC data, are now complete
- Submission based on Phase 3 data from ~45K patients demonstrating high efficacy and well-tolerated safety, including against variants
- Submissions to additional global regulatory authorities including EU, Canada and Australia expected soon
GAITHERSBURG, Md., Oct. 27, 2021/PRNewswire/ — Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializing next-generation vaccines for serious infectious diseases, today announced the completion of its rolling regulatory submission to the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) for authorization of its COVID-19 vaccine candidate. The company’s application for Conditional Marketing Authorization (CMA) marks the first submission for authorization of a protein-based COVID-19 vaccine in the United Kingdom.
“This submission brings Novavax significantly closer to delivering millions of doses of the first protein-based COVID-19 vaccine, built on a proven, well-understood vaccine platform that demonstrated high efficacy against multiple strains of the coronavirus,” said Stanley C. Erck, President and Chief Executive Officer, Novavax. “We look forward to MHRA’s review and will be prepared to deliver vaccine doses following what we anticipate will be a positive decision. We thank the clinical trial participants and trial sites in the United Kingdom, as well as the U.K. Vaccines Taskforce, for their support and vital contributions to this program.”
Novavax has now completed the submission of all modules required by MHRA for the regulatory review of NVX-CoV2373, the company’s recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M™ adjuvant. This includes preclinical, clinical, and chemistry, manufacturing and controls (CMC) data. Clinical data from a pivotal Phase 3 trial of 15,000 volunteers in the U.K. was submitted to MHRA earlier this year in which NVX-CoV2373 demonstrated efficacy of 96.4% against the original virus strain, 86.3% against the Alpha (B.1.1.7) variant and 89.7% efficacy overall, as well as a favorable safety and tolerability profile. The submission also includes data from PREVENT-19, a 30,000-person trial in the U.S. and Mexico, which demonstrated 100% protection against moderate and severe disease and 90.4% efficacy overall. NVX-CoV2373 was generally well-tolerated and elicited a robust antibody response.
Novavax expects to complete additional regulatory filings in key markets, including Europe, Canada, Australia, New Zealand, the World Health Organization and other markets around the world shortly following the U.K. submission. In the U.S., Novavax expects to submit the complete package to the FDA by the end of the year. The company continues to work closely with governments, regulatory authorities and non-governmental organizations (NGOs) in its commitment to ensuring equitable global access to its COVID-19 vaccine.
“The submission to MHRA leverages our manufacturing partnership with the Serum Institute of India, the world’s largest supplier of COVID-19 vaccines,” said Rick Crowley, Executive Vice President, Chief Operations Officer, Novavax. “In the near future, we expect to supplement this filing with supply from our global supply chain.”
Below you’ll see notes from Governor Larry Hogan’s 10/25 press conference, as tweeted by his Deputy Communications Director, Kata Hall:
• VACCINE UPDATE. Maryland has administered more than 8.3 million COVID-19 vaccines, including:
-98% of Marylanders 65+
-85.9% of Marylanders 18+
-84.9% of all eligible Marylanders 12+
• COVID-19 DATA UPDATE:
-Case rate down 39% since 9/15
-Positivity rate down 37% since 8/22
-Hospitalizations down 28% since 9/9
• COVID-19 BOOSTERS. Maryland has already administered *280,000* booster shots.
With the latest CDC approval for Moderna and J&J, nearly *1.4 million Marylanders* are now eligible to receive a booster shot.
• BOOSTER ELIGIBILITY UPDATE. Marylanders who fall into the following categories are currently eligible for a COVID-19 booster shot:

• MIX AND MATCH. Eligible Marylanders can choose *any* vaccine as a booster shot, even if it is different from what you received initially.
• ELIGIBILITY PORTAL. To help Marylanders determine their eligibility for a booster, we have launched a new portal on covidvax.maryland.gov where you can enter your information and get clear guidance.
• The data does show that the level of protection does begin to wane over time, especially for those who are immunocompromised or have comorbidities.
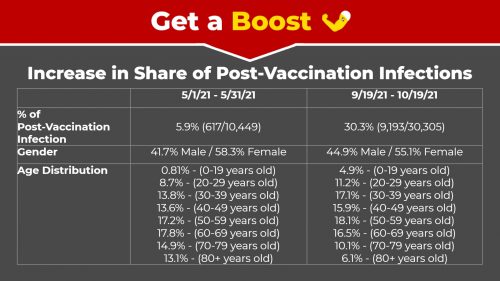
Since May, we have gradually seen an increase in post-vaccination infections:
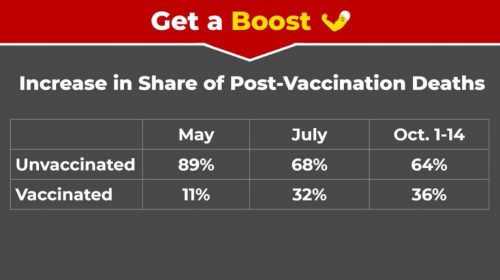
• More than half of the state’s COVID deaths over the last month are linked to hypertension and diabetes.
Cancer, obesity, and chronic lung disease are other leading comorbidities among COVID deaths in Maryland.
• State health officials have issued an advisory strongly urging people with comorbidities to get a booster dose as soon as possible.

• Visit covidvax.maryland.gov to find out where boosters are available near you.
• COVID-19 VACCINES FOR 5- TO 11-YEAR-OLDS. With CDC authorization expected as early as next week, the state is now completing final preparations to be able to vaccinate the 515,000 Maryland children who will become immediately eligible.
• Maryland health officials have begun placing orders for an initial 180,000 Pfizer doses for children.
There will be a range of options for getting 5- to 11-year-olds vaccinated, and additional updates will be provided to make sure parents have the information they need.

Updated by Montgomery County on October 15, 2021
Eligible people
These groups can currently get additional doses (booster shots and third doses):
- Residents with compromised immune systems (Pfizer or Moderna recipients, at least 28 days after second dose)
- Residents age 65 and older (Pfizer recipients only, at least six months after second dose)
- Residents age 18 and older with underlying medical conditions (Pfizer recipients only, at least six months after second dose)
- Residents aged 18-64 years who are at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting risk (Pfizer recipients only, at least six months after second dose)
We will update this page with guidance for Moderna and J&J vaccine recipients as soon as we get authorization from the CDC and State of Maryland.
Available locations
Use Maryland’s vaccine locator to find pharmacies and other providers. Your healthcare provider may have vaccine, too.
Go to a location offering Pfizer. (If you are immunocompromised, you can get Pfizer or Moderna for your additional dose).
Items to bring to your appointment
Bring these items to your additional dose appointment
- your vaccination card – see how to get a copy of your record
- personal identification – see examples of identification
You will be required to self-certify underlying medical conditions and age.
You do not need a doctor’s note or medical history.
What vaccine type should I get for my additional vaccine dose?
You should get the same mRNA vaccine (Pfizer or Moderna) that you got for your first two doses. Before you go to a clinic, confirm it has the same vaccine type you already received.
| This vaccine | is available for these additional doses |
|---|---|
| Johnson & Johnson | Not yet authorized for booster or third doses |
| Moderna | Authorized only for third doses for people with compromised immune systems |
| Pfizer | Authorized for booster shots, and third doses for people with compromised immune systems |
The CDC does NOT recommend that people with a compromised immune system who have gotten a dose of the Johnson & Johnson vaccine start a new vaccination series with Pfizer or Moderna.
Are there risks of receiving an additional vaccine dose?
Limited information exists about the risks of receiving an additional dose of vaccine. Ongoing research is looking at the safety and benefit of additional doses of COVID-19 vaccines.
So far, reactions reported after a third dose of an mRNA vaccine were similar to those for first and second doses. The most common side effects reported have been fatigue and pain at the injection site. Overall, most side effects have been mild to moderate.
Eligible people
These groups can currently get additional doses (booster shots and third doses):
- Residents with compromised immune systems ( Pfizer or Moderna recipients, at least 28 days after second dose)
- Residents age 65 and older ( Pfizer recipients only, at least six months after second dose)
- Residents age 18 and older with underlying medical conditions ( Pfizer recipients only, at least six months after second dose)
- Residents aged 18-64 years who are at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting risk ( Pfizer recipients only, at least six months after second dose)
Additional doses of J&J vaccine are not yet authorized.
Available locations
Use Maryland’s vaccine locator to find pharmacies and other providers. Your healthcare provider may have vaccine, too.
Go to a location offering Pfizer. (If you are immunocompromised, you can get Pfizer or Moderna for your additional dose).
Items to bring to your appointment
Bring these items to your additional dose appointment
- your vaccination card – see how to get a copy of your record
- personal identification – see examples of identification
You will be required to self-certify underlying medical conditions and age.
You do not need a doctor’s note or medical history.
What vaccine type should I get for my additional vaccine dose?
You should get the same mRNA vaccine (Pfizer or Moderna) that you got for your first two doses. Before you go to a clinic, confirm it has the same vaccine type you already received.
| This vaccine | is available for these additional doses |
|---|---|
| Johnson & Johnson | Not yet authorized for booster or third doses |
| Moderna | Authorized only for third doses for people with compromised immune systems |
| Pfizer | Authorized for booster shots, and third doses for people with compromised immune systems |
The CDC does NOT recommend that people with a compromised immune system who have gotten a dose of the Johnson & Johnson vaccine start a new vaccination series with Pfizer or Moderna.
Are there risks of receiving an additional vaccine dose?
Limited information exists about the risks of receiving an additional dose of vaccine. Ongoing research is looking at the safety and benefit of additional doses of COVID-19 vaccines.
So far, reactions reported after a third dose of an mRNA vaccine were similar to those for first and second doses. The most common side effects reported have been fatigue and pain at the injection site. Overall, most side effects have been mild to moderate.

